Crohn’s Disease — Overview and Worldwide Data
Crohn’s disease is a chronic, relapsing inflammatory bowel disease (IBD) that can affect any part of the gastrointestinal tract but most commonly the terminal ileum and colon. It causes transmural inflammation (inflammation through the entire bowel wall), which leads to symptoms such as abdominal pain, diarrhea, weight loss, fatigue and, in some patients, strictures, fistulae and extra-intestinal manifestations (joints, skin, eyes, liver). The exact cause is unknown but involves an interaction of genetic susceptibility, immune dysregulation, the gut microbiome, and environmental factors (smoking, diet, antibiotics and urbanization).
How it’s diagnosed and treated
Diagnosis: clinical history + blood/stool tests (inflammation markers, fecal calprotectin), endoscopy with biopsy (gold standard), and imaging (MRI/CT enterography) for small-bowel disease or complications.
Treatment goals: induce remission, maintain remission, treat complications, and improve quality of life.
Typical medication classes: aminosalicylates (limited role in Crohn’s), corticosteroids (for induction of remission), immunomodulators (azathioprine, 6-MP, methotrexate), biologic agents (anti-TNF, anti-integrin, anti-IL-12/23), and newer small molecules (JAK inhibitors, S1P modulators, etc.). Surgery is common over a lifetime for complications (stricturing, fistula, refractory disease).
Global Burden — Headline Numbers and Trends
Cases (prevalence): Global estimates range from ~3.8–6.8 million people living with IBD (which includes Crohn’s disease and ulcerative colitis), depending on the study and year. For example, a 2019 global analysis reported ~4.9 million IBD cases (GBD-based analyses), while other GBD-style summaries have reported higher historical estimates (~6.8 million in 2017 using a specific methodology). These differences reflect methodology, the year reported, and whether national registries were available.
Incidence (new cases): Global incidence has been rising overall over recent decades. One pooled analysis found global new-case counts increasing substantially from 1990 to 2021 (incidence nearly doubled in some reports), driven by rising rates in Asia, Latin America and other regions that previously had low IBD burden. High-income regions historically had the highest incidence for decades, but the fastest percentage increases are now often in newly industrialized countries.
Geographic pattern: Historically highest prevalence and incidence — North America and Western/Northern Europe. Recent trends show rapidly increasing incidence and prevalence across Asia, Latin America and parts of Africa, producing a global redistribution of disease burden. China and the USA are among the countries with the largest absolute numbers of people with IBD due to population size and rising rates.
Mortality and disability: Deaths directly attributable to IBD are relatively low compared with other chronic diseases, but IBD produces substantial disability (YLDs) and DALYs, especially where access to care is limited. Age-standardized DALY rates have shown mixed trends (some declines in high-income settings, increases in some low-/middle-income countries).
“Approximately 4.9 million people worldwide were estimated to have IBD in 2019 (GBD-type analyses).”
“Studies using earlier GBD methods reported ~6.8 million cases globally in 2017 (method-dependent estimate).”
“Incidence and absolute number of cases have generally risen since 1990, with some reports finding incidence nearly doubled between 1990 and 2021 in aggregated data.”
Who is affected?
Age: Often diagnosed in adolescents and young adults (peak onset ages late teens to 30s), but can appear at any age; pediatric IBD incidence is rising in many regions.
Sex: Slight regional variations; some datasets show a small female predominance, but patterns vary by region and study.
Risk factors: Family history/genetics (e.g., NOD2 and other loci), smoking (increases Crohn’s risk and severity), urban living/Westernized diet, antibiotics and early-life exposures, and microbiome alterations.
Why numbers vary between studies
Differences in case definitions (IBD vs. Crohn’s vs. ulcerative colitis), data sources (registry vs. claims vs. modelling), years covered, and statistical modelling choices produce different national and global totals. Many low- and middle-income countries still lack comprehensive IBD registries, so estimates there rely on modelling and are less certain. Always cite the specific study and year when quoting counts or rates. (https://www.thelancet.com/journals/langas/home)
Recent Developments & Outlook
The epidemiology is shifting: rising incidence in Asia, Latin America and parts of Africa while high-income regions remain high in absolute prevalence. This creates growing global demand for IBD diagnostics, specialist care and access to biologic/small-molecule therapies. New therapeutics continue to be approved and tested (recent approvals and trial results through 2024–2025 have expanded treatment options). (https://www.nature.com/articles/s41586-025-08940-0)
Key References
Global, regional and national burden of inflammatory bowel disease (GBD-style analyses / BMJOpen / .https://bmjopen.bmj.com/content/13/3/e065186)
The Lancet / GBD systematic analysis (2017/2019 GBD publications describing global estimates).https://www.thelancet.com/journals/langas/home
Nature review / 2025 synthesis on global evolution of IBD (trends and geographic shifts).
Regional reviews (e.g., epidemiology in Asia).
Patient-facing stats and background (Crohn’s & Colitis Foundation).
References on Crohn’s Disease
Duerr, R. H., Taylor, K. D., Brant, S. R., et al. (2006). A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science, 314(5804), 1461–1463. (Landmark GWAS discovery of IL23R in Crohn’s disease)
Liu, J. Z., van Sommeren, S., Huang, H., et al. (2015). Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nature Genetics, 47(9), 979–986. (Major GWAS meta-analysis expanding known IBD loci)
Collaborators of the Global Burden of Disease Study (2019). Global, regional, and national burden of inflammatory bowel disease, 1990–2019. The Lancet Gastroenterology & Hepatology, 4(10), 795–808. (Comprehensive GBD burden analysis)
Kaplan, G. G., & Windsor, J. W. (2021). The global evolution of inflammatory bowel disease. Nature Reviews Gastroenterology & Hepatology, 18(1), 56–66. (Key review of global epidemiology trends, rising incidence in Asia/Latin America)
Asthma
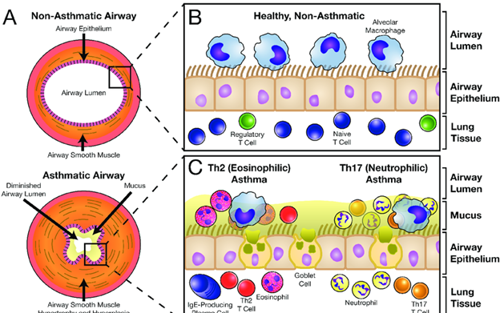
reference for the picture: https://www.researchgate.net/figure/The-Normal-and-Asthmatic-Airway
What is asthma
Asthma is a chronic inflammatory airway disease characterized by variable airflow limitation and bronchial hyper-responsiveness. Typical symptoms are wheeze, breathlessness, chest tightness and cough that vary over time and in intensity. Inflammation and airway remodeling can lead to exacerbations and (if poorly treated) long-term lung function loss.
Key causes / risk factors
Type 2 (allergic) inflammation is common, but non-type-2 phenotypes exist.
Risk factors: family history, atopy/allergies, viral respiratory infections (especially early life), air pollution, tobacco smoke, obesity and occupational exposures.
How it’s diagnosed
Clinical history + demonstration of variable airflow limitation (spirometry with bronchodilator response or peak flow variability).
Other tests: bronchial challenge tests, FeNO (fractional exhaled nitric oxide) for eosinophilic inflammation, allergy testing and chest imaging when needed. Management is guided by symptom control, exacerbation history, lung function and biomarkers where available.
Main treatments (overview)
Controller therapy: inhaled corticosteroids (ICS) — cornerstone for reducing inflammation and preventing exacerbations.
Reliever therapy: short-acting β2-agonists (SABA) historically, but recent strategy updates favor ICS–formoterol as preferred reliever in many patients to reduce exacerbation risk.
Add-ons: long-acting bronchodilators (LABA), LAMA, leukotriene modifiers, biologics (anti-IgE, anti-IL-5, anti-IL-4/13, etc.) for severe eosinophilic or allergic phenotypes; and non-pharmacologic measures (vaccination, smoke avoidance, trigger control). These recommendations are reflected in the 2024 GINA strategy.
Worldwide impact — headline numbers & trends
Rough global prevalence / cases: recent Global Burden of Disease (GBD) analyses and allied syntheses estimate ~260 million people living with asthma in recent years (GBD/HealthData estimates around 2019–2021). This is a commonly cited ballpark figure for the global burden.
Deaths and DALYs: WHO and GBD reports indicate hundreds of thousands of deaths annually in earlier estimates (WHO cited ~400k deaths in older summaries) and tens of millions of DALYs (e.g., ~21–25 million DALYs attributed to asthma in GBD-era summaries). Absolute death totals and DALYs vary by year and method.
Geography & trends: historically highest prevalence and burden were reported in high-income regions (North America, Western Europe, Australasia), but trends show shifting and heterogeneous patterns: some high-income countries have stable or declining age-standardized rates while many low- and middle-income countries (LMICs) — especially parts of Asia, Africa and Latin America — show rising incidence, large numbers of undiagnosed or undertreated cases, and significant gaps in access to inhaled corticosteroids and other care. Recent analyses covering 1990–2021 describe falling age-standardized prevalence per 100,000 in some datasets but rising absolute case counts and ongoing inequities.
Children and adolescents: Asthma is the most common chronic disease in children; pediatric asthma incidence and the proportion of undiagnosed cases remain high in many countries, with studies showing many adolescents in parts of Africa and Asia remain undiagnosed and untreated.
Burden drivers & disparities: poor control, lack of access to regular inhaled corticosteroids, air pollution, tobacco exposure, and socioeconomic disparities drive higher rates of exacerbations, emergency visits and deaths in disadvantaged populations.
Practical facts you can quote
“About 260 million people worldwide are estimated to have asthma (GBD/HealthData estimates for recent years).”
“Asthma causes tens of millions of DALYs globally and was responsible for hundreds of thousands of deaths in prior global summaries; burden is concentrated where diagnosis and long-term controller treatment are limited.”
“Global clinical guidance (GINA 2024) updates recommend earlier use of anti-inflammatory relievers (ICS–formoterol) and widened access to effective controller therapies to reduce exacerbations and deaths.”
Key References
WHO — Asthma fact sheet. (WHO overview of burden, causes, prevention/control).
GBD / HealthData summary for asthma (1990–2021 analyses) — global/regional/national prevalence, incidence and DALYs. (HealthData / GBD pages & accompanying Lancet/peer-reviewed analyses).
Lancet eClinicalMedicine (2024): “Global, regional, national burden of asthma from 1990 to 2021…” (recent peer-reviewed analysis of trends).
GINA 2024 Strategy Report — up-to-date global clinical guidance on management and prevention (PDF available).
GlobalAsthmaReport.org / Global Asthma Network — synthesis resources and country/regional insights on diagnosis gaps and access.
Correlation between Crohn's and Asthma
Shared or Reported Pleiotropic Genes Between Asthma & Crohn’s Disease
Gene / Locus | Function / Pathway | Evidence in Asthma | Evidence in Crohn’s / IBD | Notes on Overlap |
|---|---|---|---|---|
ORMDL3 / GSDMB (17q12–21 region) | ER stress, sphingolipid metabolism, airway inflammation | Strong, consistent GWAS association (esp. childhood asthma) | Some signals in IBD GWAS / regulatory pleiotropy reported | Strong asthma locus, occasional IBD signal; tissue-specific effects |
DENND1B | Regulates clathrin-mediated endocytosis, immune signaling | GWAS locus in asthma | Reported in IBD pleiotropy reviews | Candidate shared locus, not strongly replicated in IBD |
SMAD3 (TGF-β signaling) | Immune regulation, tissue remodeling | Associated with asthma traits, airway inflammation | Implicated in Crohn’s GWAS and immune regulation | Pathway plausibility (immune regulation, remodeling) |
SLC22A4 / SLC22A5 (IBD5 region, 5q31) | Solute transporters, immune regulation | Linked to atopy/asthma in older studies | Classic Crohn’s/IBD locus (IBD5) | Historical overlap; modern GWAS show stronger signal in IBD |
PTPN2 | Tyrosine phosphatase, immune regulation | Associated with immune/allergic traits in some studies | Strong IBD gene | Shared immune signaling regulation |
IL23R / IL12B (Th17 pathway) | Cytokine signaling, Th17 immunity | Weak/limited in asthma | Strong Crohn’s / IBD association | Pathway overlap more than direct asthma gene |
Big-Picture Findings
Genome-wide correlation is limited: Large 2024 genetically informed studies (UK Biobank, cross-trait GWAS) found little robust evidence for a broad shared genetic origin between asthma and IBD. Most genetic risk is disease-specific.
Pleiotropic loci exist, but they are few, and their effects often differ by tissue context (airway vs gut).
References (Key)
Comorbidity Between Inflammatory Bowel Disease and Asthma and Allergic Diseases: A Genetically Informed Study (2024) — finds little genome-wide overlap.
Inflammatory bowel disease and airway diseases (review) — summarizes loci like DENND1B, SMAD3, ORMDL3, SLC22A4/5 as shared.
Evidence for Significant Overlap Between Common Risk Variants (PLOS GWAS analysis) — reports pleiotropic immune-regulatory loci.
Shared genetic architecture across multiple traits (cross-trait GWAS atlas, 2024–2025) — shows a handful of pleiotropic regions but overall limited asthma–IBD overlap.
Asthma GWAS meta-analyses (GINA, ORMDL3/17q12–21 region) and Crohn’s GWAS (IL23R, NOD2, IBD5)
Additional References
Li, X., et al. (2024). Comorbidity between inflammatory bowel disease and asthma and allergic diseases: A genetically informed study. PMC Article / PubMed (UK Biobank cross-trait analysis showing limited overlap).
Kelsen, J. R., Baldassano, R. N., & Conrad, M. A. (Review). Inflammatory bowel disease and airway diseases: Epidemiology, genetics, and immunology. Frontiers in Immunology. Summarizes loci such as DENND1B, SMAD3, SLC22A4/5, and ORMDL3 as shared candidates.
Ellinghaus, D., et al. (2012). Evidence for significant overlap between common risk variants for Crohn’s disease and asthma. PLoS Genetics, 8(9), e1002768. https://doi.org/10.1371/journal.pgen.1002768 (Reports pleiotropic immune-regulatory loci across Crohn’s and asthma).
Cross-trait GWAS Atlas Consortium (2024). Shared genetic architecture across immune-mediated and allergic diseases. Nature Genetics (online ahead of print). (Maps pleiotropic loci and shows limited asthma–IBD overlap, except at select immune loci).
Moffatt, M. F., et al. (2010). A large-scale, genome-wide association study of asthma identifies multiple susceptibility loci. Nature Genetics, 42(12), 1084–1093. (Classic identification of ORMDL3/GSDMB locus at 17q12–21 in asthma).
Duerr, R. H., et al. (2006). A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science, 314(5804), 1461–1463. (Landmark Crohn’s GWAS identifying IL23R; central to Th17 pathway).
Moffatt, M. F., Gut, I. G., Demenais, F., et al. (2010). A large-scale, genome-wide association study of asthma identifies multiple susceptibility loci. Nature Genetics, 42(12), 1084–1093. (Identifies ORMDL3/GSDMB at 17q12–21 as a major asthma locus).
Global Initiative for Asthma (GINA). (2024). Global Strategy for Asthma Management and Prevention. https://ginasthma.org (Most up-to-date international guidelines on diagnosis and treatment).
Global Burden of Disease Study Asthma Collaborators. (2024). Global, regional, and national burden of asthma from 1990 to 2021: a systematic analysis. eClinicalMedicine. (Latest GBD-based epidemiology study).
World Health Organization. (2024). Asthma fact sheet. https://www.who.int/news-room/fact-sheets/detail/asthma (WHO global data on prevalence, deaths, and control gaps).
